- Analytical methods
- PLA 3.0
- Try & Buy
- PLA 3.0 Academy
- Support
- Company
bioMérieux Endotoxin Detection Assay Package

Load and watch this YouTube video now.

Analyze the bioMérieux assay kits - developed in collaboration and part of something bigger
Perform an endotoxin test based on recombinant Factor C (rFC), a synthetic alternative to the naturally occurring reagents derived from horseshoe crabs, such as those offered by bioMérieux in their kits:
- ENDOLISA®
- ENDOZYME® II GO
- ENDOZYME® II GO STRIPS
and control the Tecan Fluent® Automation Workstation with our Tecan Magellan™ Import Module for bioMérieux Endotoxin Detection Assays.
The bioMérieux Endotoxin Detection Assay Package has been developed collaboratively between bioMérieux, Tecan and Stegmann Systems as an extension of PLA 3.0.
PLA 3.0 is the statistical analysis software which is used by all top 100 companies in the regulated industries, particularly in pharmaceuticals, biotechnology, drug discovery, and other life sciences sectors. It includes a wide range of functionalities and provides an extensive array of statistical tools. Which means that your company does not need any additional software, as PLA 3.0 covers your entire development lifecycle out of the box.
Effortless precision, seamless documentation
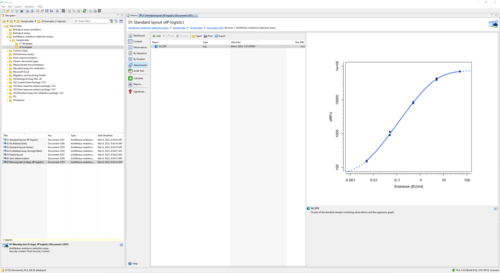
Effortlessly streamline your assay analysis process with intuitive configuration options and adjustable templates. Use our state-of-the-art software to accurately fit linear or 4-parameter logistic models to your reference standard, enabling precise calculations of sample activity and spike recovery from positive product controls. Easily optimize instrument sensitivity by calculating ideal amplification while ensuring assay validity through comprehensive testing of regression parameters, recovery, coefficient of variation, and blank control development. Seamless documentation of instrument settings and operator information for a smooth and efficient workflow are a given.
Elevate your reporting: Export your results to PDFs with ease
Simplify your reporting process by effortlessly creating tailored PDF reports. Additionally, take advantage of our data export capabilities for external data processing.
Full reports
-
Sample Report for a bioMérieux endotoxin assay using the 4-parameter logistic model 77 KB
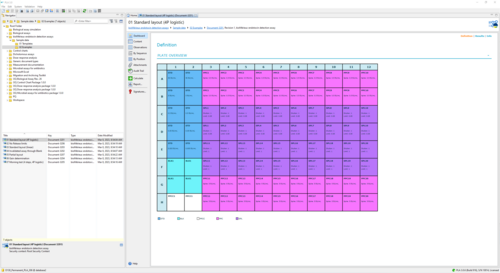
This report shows an example of the execution of an ENDOZYME® II GO assay. It uses a Standard with 5 dilution steps (50 - 0.005 EU/mL) fitted via a 4-parameter logistic curve. The plate is filled with 20 samples. One half uses a release limit of 0.05, the other half a limit of 1. -
Sample Report for a bioMérieux endotoxin assay using the linear model 76 KB
This report shows an example of the execution of an ENDOZYME® II GO assay. It uses a Standard with 4 dilution steps (5 - 0.005 EU/mL) fitted via a linear curve. The plate is filled up with 20 samples. The upper half of the samples uses a 1:2 predilution. The lower half is not prediluted. A release limit of 0.05 is applied to each sample. -
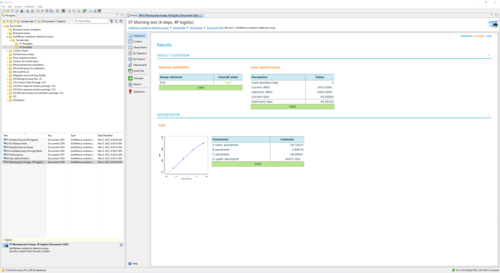
Sample Report for a bioMérieux endotoxin assay executing the Morning test 34 KB
This report shows the execution of the Morning test for an endotoxin detection assay. The plate setup uses one strip, and columns 1 & 2 are filled with Standard concentrations. Besides the normal evaluation of the curve fit, it is checked whether the current gain of the instrument is correctly calibrated. This is verified by comparing the CV of the measured dRFUs on the chosen Standard concentration (=0.5 EU/mL) to an upper bound. In the example, the gain succeeds the test. In fact, its result and validity of the gain optimization does not influence assay validity at all and can be seen as an informative remark for the user.
Application example
Endotoxins are part of the cell membrane from gram-negative bacteria. When released into a host organism after cell decay, endotoxins can cause serious pyrogenic effects. Detecting the amount of endotoxin in pharmaceutical products, which enter the human bloodstream, is therefore a mandatory step performed by laboratories.
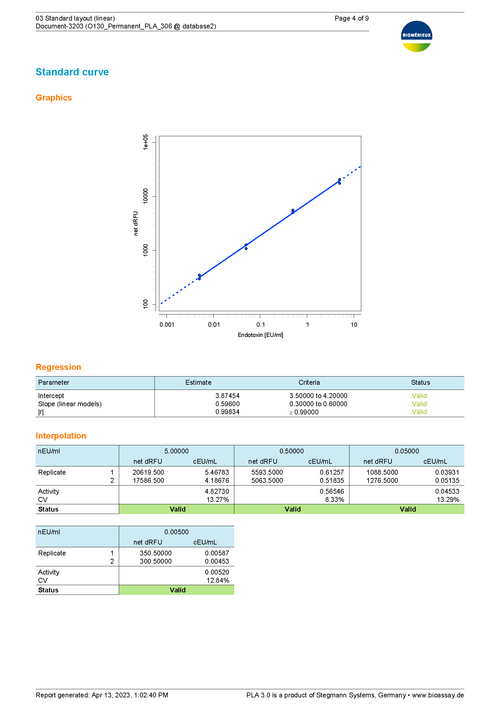
The bioMérieux endotoxin detection assay document provides all necessary tools to analyze endotoxin concentration as suggested by bioMérieux. The add-on comes with templates to support the 96-well test kits ENDOZYME® II (Go) and ENDOLISA® from bioMérieux. You can also define custom assay templates to use with your own assay configuration. Choose between linear and non-linear regression models to fit data of your reference standard.
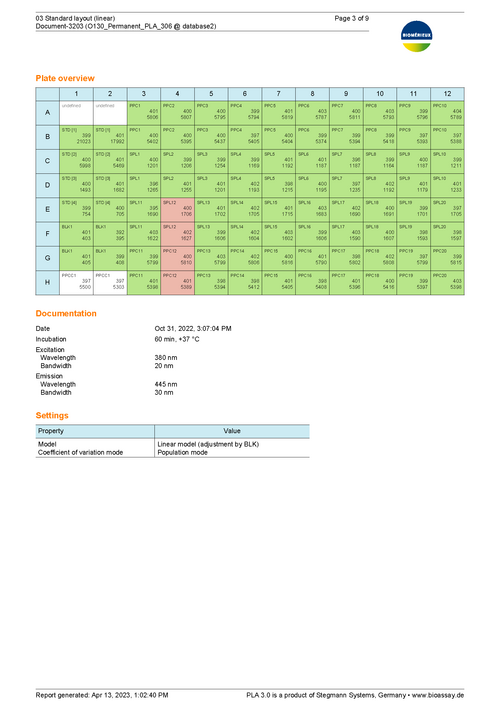
Prepare test samples by adding information about the threshold value of endotoxin concentration or the spike of the respective positive product control (PPC). Further add blank controls to use them as the baseline for the linear model approach and to determine assay validity.
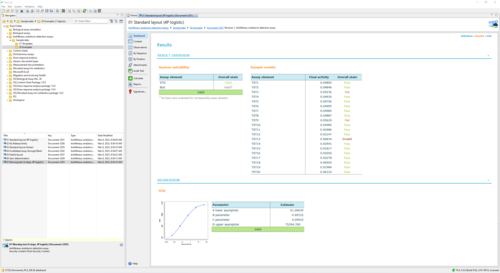
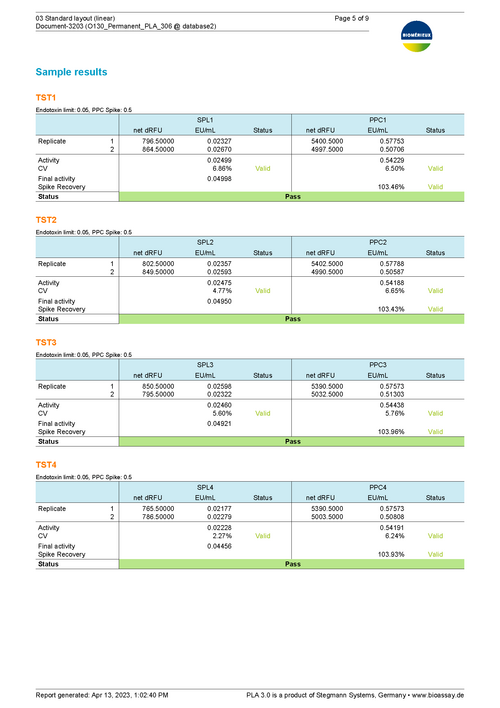
Results show the activity of test samples in relation to the reference standard, and the recovery rate of spiked PPCs. Assess the validity of your assay with configurable tests on regression parameters, PPC recovery rate, blank control development, or the variation in estimated endotoxin concentration.
The bioMérieux endotoxin detection assay document also allows for the calculation of the optimum gain to qualify plate reader sensitivity. Create print-ready PDF reports or use the CSV format for further processing.