-
PLA 3.0
- PLA 3.0 Virtual booth
- ---
- Explore PLA 3.0
- Compliance features
- Deployment
- ---
-
Experiments
- Parallel-line potency assays
- Parallel logistic potency assays (3PL, 4PL, 5PL)
- Slope ratio potency assays
- Quantal response potency assays
- Interpolation analysis
- Effective-concentration calculation (ECn)
- Spike-and-recovery analysis
- Cylinder-plate assays
- Turbidimetric assays
- ---
- Analyze the endotoxin concentration in a substance
- Import your data
-
Analyze your data with PLA 3.0
- Analyze Biological Potency Assays
- Analyze quantal response assays
- Analyze the dose-response relationship
- Analyze the endotoxin concentration in a substance
- Develop Equivalence margins
- Determine the potency of antibiotics
- Perform a curve comparisons
- Perform a Linearity-of-dilution assessment
- Perform a sophisticated statistical process control
- Perform combination calculations
- Advanced analysis
- Monitoring
- Supporting add-ons
- ---
- Event Calendar
- News
- Newsletter
- Get started
- Downloads
- Company
Combination calculation
To achieve ‘reportable values’ for your assays, you need to perform combination calculations of your independent biological assays. The combination calculation of PLA 3.0 aggregates the data of independent assay runs into a combination calculation.
Combination of Independant Assay Results
The European Pharmacopoeia and the US Pharmacopeia describe different methods of combination calculations for independent assay results to obtain reportable values for the potency. PLA supports all different weighting methods for combination calculations. PLA also supports automatic data aggregation of independent assay data.
- Unweighted Combination
- Homogeneously Weighted Combination
- Heterogeneously Weighted Combination according to European Pharmacopoeia, chapter 5.3
- Heterogeneously Weighted Combination according to US Pharmacopeia <111>
- Heterogeneously Weighted Combination according to US Pharmacopeia <1034>
Grouping of Calculations
PLA 3.0 also supports grouping of combination calculations.
Example: consider two independent quantitative response assays with two test samples in each assay: Assay 1 contains test samples for Product A and Product B. Assay 2 contains test samples for the same products. In a grouped analysis, you will receive two separate combination calculations. One for Product A as well as one for Product B.
Test System
You can set up acceptance tests for the calculation results of the combination calculation.
Example Combination of Assay Results with PLA 3.0
-
Example Combination of Assay Results with PLA 3.0 28 KB
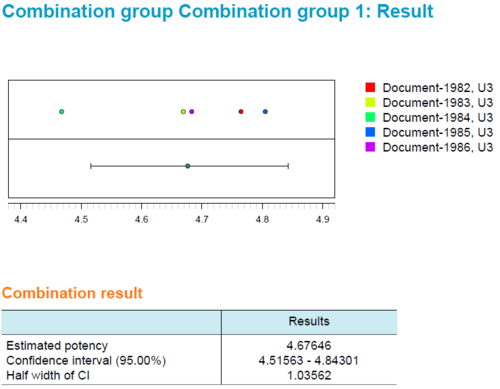
The report contains a combination calculation for relative potency results, based on European Pharmacopoeia 6.0, chapter 5.3. As described in European Pharmacopoeia up to the 9th edition, 'Heterogeneous Weighting' was selected as the combination method. Page 1 is a typical PLA 3.0 report title page with basic information on the document and the database. Information on electronic document signatures would be also shown here. Page 2 shows a table with the general properties of the whole document. The source data is taken from reference documents (assays), calculated using PLA 3.0. The data is structured into two groups. The results of each group are listed separately. Page 3 shows the results for group 1. Page 4 shows the results for group 2. For each group, the results of the source data were combined according to the configured calculation method. The top-most table in each group contains details on the source data. The following table lists the values and a set of resulting calculations. The 'Tests on combination result' section lists the results of the suitability tests. In the sample document, a test for a combined relative potency value was set up. The 'Calculation of the combined relative potency and 95% confidence limits' section shows the corresponding test results. The plot shows the input values and the results.
Microbial Assays for Antibiotics Package
The Microbial Assays for Antibiotics Package provides its own document type for combination calculations of independent cylinder-plate or turbidimetric assays. Aggregate such assays into the document to obtain the estimated combined potency for each combination group. Acceptance tests cover the specific values of this method.
Sample Report for a combination of microbial assays with PLA 3.0
-
Sample Report for a combination of microbial assays 49 KB
Example combination of microbial assay documents. Cylinder-plate assays (based on USP <81>) are aggregated to determine the combined potency. In addition, test samples from the original assay documents are put into combination group 1 and 2 respectively. This results in a separate analysis for each group. The test system includes tests on the combined potency, the confidence interval of the estimated combined potency, and the CV (%) of the absolute potency values.