-
PLA 3.0
- PLA 3.0 Virtual booth
- ---
- Explore PLA 3.0
- Compliance features
- Deployment
- ---
-
Experiments
- Parallel-line potency assays
- Parallel logistic potency assays (3PL, 4PL, 5PL)
- Slope ratio potency assays
- Quantal response potency assays
- Interpolation analysis
- Effective-concentration calculation (ECn)
- Spike-and-recovery analysis
- Cylinder-plate assays
- Turbidimetric assays
- ---
- Analyze the endotoxin concentration in a substance
- Import your data
-
Analyze your data with PLA 3.0
- Analyze Biological Potency Assays
- Analyze quantal response assays
- Analyze the dose-response relationship
- Analyze the endotoxin concentration in a substance
- Develop Equivalence margins
- Determine the potency of antibiotics
- Perform a curve comparisons
- Perform a Linearity-of-dilution assessment
- Perform a sophisticated statistical process control
- Perform combination calculations
- Advanced analysis
- Monitoring
- Supporting add-ons
- ---
- Event Calendar
- News
- Newsletter
- Get started
- Downloads
- Company
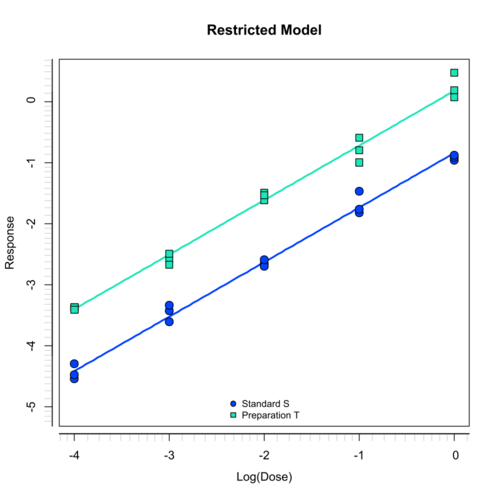
Parallel-line potency assays
A parallel-line assay (PLA) is the classical method to calculate a relative potency for a dilution assay. It is a linear fit which covers only the (near-) linear portion of the dose-response relationship without their asymptotes.
PLA 3.0 supports parallel line assays along with additional functionality to determine acceptable regions of the dose-response curve (configuration optimization).
The parallel-line method is a robust biostatistical analysis method based on D.J. Finneys Statistical Methods in Biological Assay, 3. edition, 1978. It is supported by the European Pharmacopoeia and the US Pharmacopeia.
PLA 3.0 allows you to carry out the regression for each standard/sample combination in your bioassay separately or at once.
Use this approach for all typical bioassays, such as
- binding assays (for example ELISA),
- enzyme activity assays (for example spectrophotometric, fluorometric or radiometric assay),
- cell based assays (for example cell proliferation, cytotoxicity, or cell death assay) or
- cell and gene therapy assays.
Example report of a Parallel-line assay analyzed with PLA 3.0
-
Example report of a Parallel-line assay analyzed with PLA 3.0 71 KB
Based on European Pharmacopoeia, 5th Edition (2005), Chapter 5.3 5.1.4 Five-dose multiple assay with completely randomised design - An in-vitro assay of three hepatitis B vaccines against a standard Remarks: Multiplex analysis