-
PLA 3.0
- PLA 3.0 Virtual booth
- ---
- Explore PLA 3.0
- Compliance features
- Deployment
- ---
-
Experiments
- Parallel-line potency assays
- Parallel logistic potency assays (3PL, 4PL, 5PL)
- Slope ratio potency assays
- Quantal response potency assays
- Interpolation analysis
- Effective-concentration calculation (ECn)
- Spike-and-recovery analysis
- Cylinder-plate assays
- Turbidimetric assays
- ---
- Analyze the endotoxin concentration in a substance
- Import your data
-
Analyze your data with PLA 3.0
- Analyze Biological Potency Assays
- Analyze quantal response assays
- Analyze the dose-response relationship
- Analyze the endotoxin concentration in a substance
- Develop Equivalence margins
- Determine the potency of antibiotics
- Perform a curve comparisons
- Perform a Linearity-of-dilution assessment
- Perform a sophisticated statistical process control
- Perform combination calculations
- Advanced analysis
- Monitoring
- Supporting add-ons
- ---
- Event Calendar
- News
- Newsletter
- Get started
- Downloads
- Company
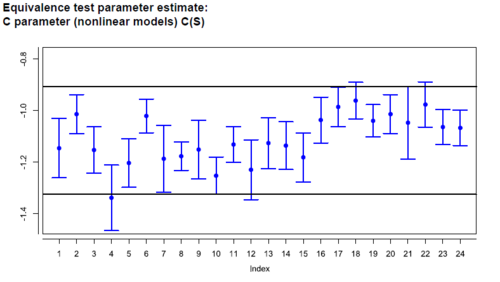
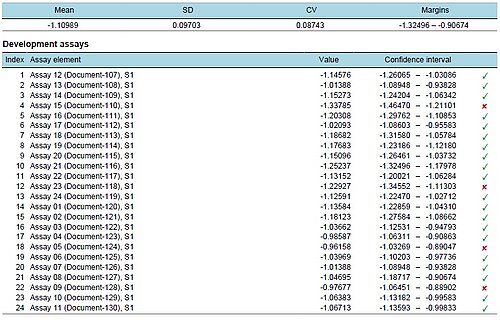
Equivalence margin development for similarity testing
The development of equivalence margins for use according to the US Pharmacopeia is a challenging task. PLA 3.0 supports you with development assays which are used to develop the margins and verification assays, that verify your test strategies. Test strategies are selections of available predefined or calculated margins that offer the possibility to include different strategies. The strategies can be visualized. The system is going to simulate a certain number of acceptable assays for every defined strategy to support the visual verification of the strategies. Besides this, the equivalence margin development offers a broad variety of equivalence tests.
Similarity Testing
Similarity testing is the key to success in highly precise biological assays. PLA 3.0 focusses on the challenge of margin development with an automated method, that turns the development of candidate equivalence margins into an easy task.
Example of an Equivalence margin development report in PLA 3.0
-
Example of an Equivalence margin development report with PLA 3.0 679 KB
The report is split into five sections. The first section provides an overview about the source assays. The second section shows the developed equivalence margins. Section three contains an overview about the utilized test strategies. In section four, these strategies are verified against each development and verification assay. And section five visualizes the behaviour of the strategies by running simulations for acceptable assay systems.